Allan Griff, consulting chemical engineer, columnist for PlasticsToday, and self-professed realist, came across an article in MIT News riddled with scientific falsehoods. He shares his thoughts.
MIT News sent me a report on research involving zeolites, porous minerals used to make propane from scrap (recycled) polyolefins with a cobalt catalyst. I was surprised by how scientifically wrong and misleading the article was, especially considering its origin at MIT.
Porous zeolites are well known. If researchers can use their pore size to produce 3-carbon molecules (propane), that's newsworthy. But it begs the question of how much 1-carbon (methane) and 2-carbon (ethane) get through and what you do with them.
The article also implies that recyclable polyolefins are useless pollutants, which is wrong because they are not toxic in their normal solid form — very strong C-C bonds, long chains, low reactivity. I'd worry more about the toxicity of cobalt than the plastics.
Toxicity of solid plastics is a popular image based on the human need to resist science so that we can believe in the impossible, which goes back to the comforts of infancy when nothing can be explained.
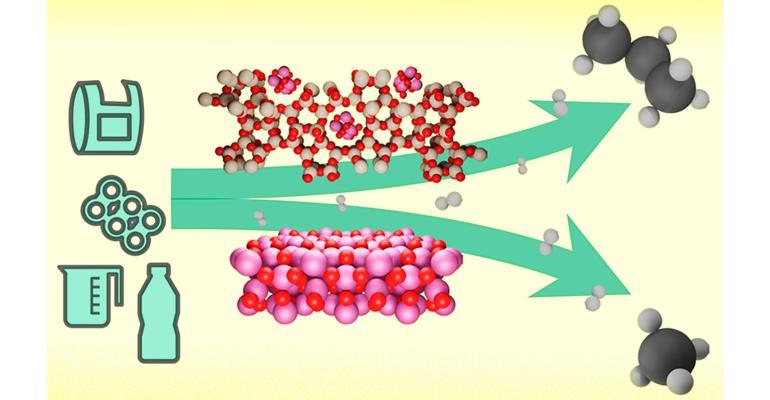
The article mixes up PET and PE and includes a drawing (above) of a soda bottle, which is made from PET, chemically very different from polyolefins and already valuably recycled. Not irrelevant, as it appeals to people who see lots of plastic bottles and think all plastics are harmful.
The drawing is also misleading as it shows the feed of a ringed (aromatic) plastic and the making of propylene, not propane. Propylene may be worth more than propane and doesn't need added hydrogens. The drawing also shows production of methane, which isn't wanted, especially in the air.
The article states that the economics to make propane and sell it are promising, but the authors give neither investment nor operating nor sales/price data. And there’s nothing on energy needs in kilowatt-hours, which may make the process less attractive to many environmental-minded people. You need to break a lot of those strong C-C bonds to break the polymer chain, a basic flaw in much advanced/chemical recycling except some pyrolysis.
Lastly, or actually first, the article invokes the popular image of plastics in humans (and fish), ignoring the impossibility of digestion or circulation. The particles are far too big to penetrate the gut wall and then circulate through a network of capillaries. And how much matters, as I often say. Discarded fishnets may be harmful to aquatic creatures, but so is catching fish and eating them.
Yet, many people still want to believe that micro-plastics are inside us to support their need to resist science, which deprives them of the comfort of miracles. They are quick to label plastic toxic because it is:
●unnatural (but earthquakes and viruses are natural);
●a chemical (but everything is made of chemicals, including water, air, and us);
●changeable (but so is weather and our bodies);
●synthetic (but so are many medicines and foods);
●corporate (but corporations are creative and keep prices down when responsibly regulated).
What we really fear is ourselves — humanipulation.
It isn't only the unscientific masses who think this way. Our own industry is investing in efforts to stop "plastics pollution" as are the politicians who correctly see such myth-understanding as doing what the voters want.
Waste is a separate problem from pollution, and our plastics industry can and should reduce its losses. But let’s not forget that plastics help reduce other waste — food, energy, water — and prevent pathogen growth and infection, but cause none.
Plastics are relatively harmless but people want them to be bad? Yes, and now maybe you see why.
Post time: Dec-09-2022